
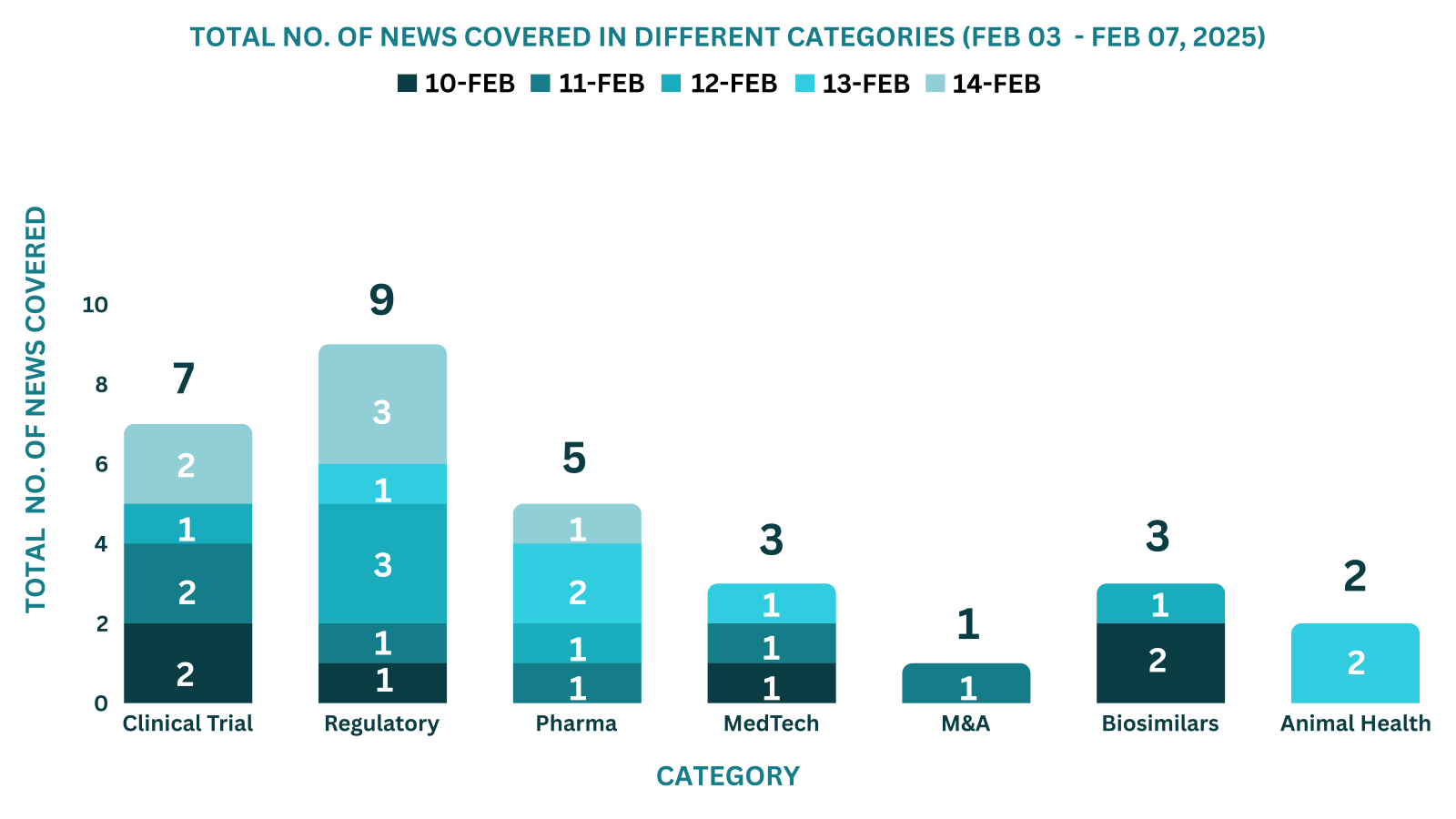
PharmaShots Weekly Snapshots (February 10th, 2025 – February 14th, 2025)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Animal Health & Biosimilars. Check out our full report below:

Eli Lilly Reveals VIVID-2 Study Data of Omvoh to Treat Crohn's Disease
Read More: Eli Lilly
Regeneron Reveals Data from Extended P-III (PULSAR) Trial of Eylea HD for Wet Age-related Macular Degeneration
Read More: Regeneron
BMS Reports Topline Data from P-II (TRANSCEND FL) trial of Breyanzi for R/R Indolent B-cell non-Hodgkin lymphoma
Read More: BMS
Boehringer Ingelheim Reports Topline Data from P-III (FIBRONEER-ILD) Study of Nerandomilast for Progressive Pulmonary Fibrosis (PPF)
Read More: Boehringer Ingelheim
Pfizer and Astellas Report Follow-up Data from P-III (KEYNOTE-A39/EV-302) Trial of Padcev + Keytruda for Locally Advanced or Metastatic Urothelial Cancer (la/mUC)
Read More: Pfizer and Astellas
Pfizer Reports Data from P-III (TALAPRO-2) Trial of Talzenna + Xtandi for Metastatic Castration Resistant Prostate Cancer (mCRPC)
Read More: Pfizer
Sanofi and J&J Discontinue P-III (E.mbrace) Study of ExPEC9V for Invasive E. coli Diseases
Read more: Sanofi and J&J

Abbvie Reports the US FDA Approval for Emblaveo to Treat Complicated Intra-Abdominal Infections (cIAI)
Read More: AbbVie
LIB Therapeutics Reports the US FDA’s BLA Acceptance for Lerodalcibep to Reduce LDL-Cholesterol in Cardiovascular Patients
Read More: LIB Therapeutics
Regeneron Reports US FDA’s Acceptance of Resubmitted BLA for Linvoseltamab to Treat R/R Multiple Myeloma (MM)
Read More: Regeneron
SpringWorks Therapeutics Receives US FDA Approval for Gomekli to Treat Neurofibromatosis Type 1 Associated Symptomatic Plexiform Neurofibromas (NF1-PN)
Read More: SpringWorks Therapeutics
BridgeBio’s Beyonttra (Acoramidis) Gets the EU approval to Treat Transthyretin Amyloidosis Cardiomyopathy (ATTR-CM)
Read More: BridgeBio
Pfizer Reports the US FDA Approval of Adcetris Regimen to Treat R/R Diffuse Large B-Cell Lymphoma (DLBCL)
Read More: Pfizer
CSL Behring Receives the EC Approval for Andembry as a Prophylactic Treatment of HAE
Read More: CSL Behring
Bayer Submits MAA of Finerenone to Japan’s MHLW for Heart Failure (HF)
Read More: Bayer
Galderma’s Nemluvio (Nemolizumab) Secures the EU Approval to Treat Moderate-To-Severe Atopic Dermatitis (AD) and Prurigo nodularis (PN)
Read More: Galderma

Eli Lilly Acquires OLX75016 from OliX Pharmaceuticals for Metabolic-Associated Steatohepatitis
Read More: Eli Lilly
Alloy Therapeutics Enters into a Multi-Year Strategic Collaboration with Pfizer to Develop New Antibody Discovery Platform
Read More: Alloy Therapeutics and Pfizer
AbbVie Collaborates with Xilio Therapeutics to Develop Next-Generation Tumor-Activated Immunotherapies
Read More: AbbVie and Xilio Therapeutics
Royalty Pharma Partners with Biogen to Develop Litifilimab
Read More: Royalty Pharma and Biogen
IDEAYA Enters Into an Additional Clinical Study Collaboration and Supply Agreement with Gilead Sciences
Read More: IDEAYA and Gilead Sciences

AliveDx Seeks US FDA’s 510(k) Clearance for MosaiQ AiPlex Multiplex Microarray
Read More: AliveDx
Ibex Medical Analytics Receives the US FDA’s 510(k) Clearance for Ibex Prostate Detect
Read More: Ibex Medical Analytics
Johnson & Johnson MedTech Launches Cereglide 92 Catheter System in the US for Acute Ischemic Stroke
Read More: Johnson & Johnson

Novartis to Acquire Anthos Therapeutics for ~$3.1B
Read More: Novartis and Anthos Therapeutics

Dalan Animal Health Unlocks the Potential of Innate Immunity with its Vaccine Platform
Read More: Dalan Animal Health
Boehringer Ingelheim Launches Semintra in the EU to Treat Feline Hypertension
Read More: Boehringer Ingelheim

Bio-Thera Reports the EMA’s MAA Acceptance of BAT2506 (Biosimilar, Simponi)
Read More: Bio-Thera
Dr. Reddy’s Partners with Henlius to Commercialize HLX15 (Biosimilar, Darzalex & Darzalex Faspro) in the US & EU
Read More: Dr. Reddy’s and Henlius
Celltrion Secures the US FDA’s Approval for Avtozma IV/SC (Biosimilar, Actemra)
Read More: Celltrion
Related Post: PharmaShots Weekly Snapshots (February 3rd, 2025 – February 7th, 2025)
Tags

Ridhi is an avid secondary researcher who follows trends in the biopharmaceutical and healthcare sectors to curate engaging content for the global audience. She works as a news editor at PharmaShots and loves to read books and explore new destinations.














